Abstract
Introduction: Diffuse large B-cell lymphoma (DLBCL) is an aggressive form of non-Hodgkin's lymphoma (NHL), encompassing 30-58% of all NHL cases and approximately 60,000 cases annually in the United States of America (US) alone (Tilly et al, 2015; Zolkefli, 2017). The treatment (tx) landscape for DLBCL has significantly expanded, with novel targeted therapies, immunotherapies in addition to chemotherapy and chimeric antigen receptor therapy (CAR-T) (Bachanova et al, 2020). The importance of shared decision-making in cancer tx selection is well recognized; yet, we lack data on patient preferences regarding DLBCL tx, and existing research on tx factors in other disease areas, such as regimen intensity, has shown mixed results (Van Hoogdalem et al, 2019; Rummel et al, 2017; Rubin and Peyrot, 1999).Thus, we aimed to examine patient preferences of tx characteristics across the DLBCL landscape.
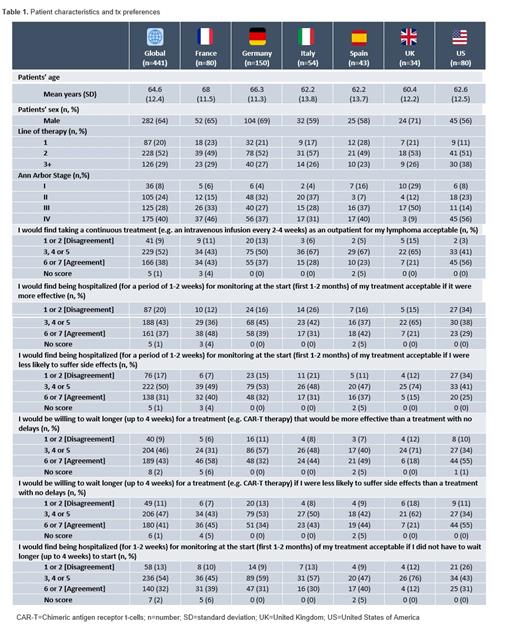
Methods: Data were drawn from the Adelphi DLBCL Disease Specific Programme™, a point-in-time survey of hematologists, hemato-oncologists, oncologists, and their DLBCL patients conducted in France (FR), Germany (DE), Italy (IT), Spain (SP), the United Kingdom and the US between Jan-May 2021. Eligible patients were asked by their consulting physician to voluntarily complete a patient self-completion form (PSC) capturing demographics and preferences encompassing their DLBCL tx options. Patient tx preference questions were presented in a simplified manner to reduce the risk of misinterpretation. Patients' level of acceptance for characteristics of tx options, such as the patients' willingness to "be hospitalized" or "wait longer" for a more effective tx, were rated using a 7-point Likert scale (1=total disagreement; 7=total agreement). A Likert score of 6-7 was considered "agreement", whilst a Likert score of 1-2 was considered "disagreement".
Results: Data analysis were conducted on 441 DLBCL patients (FR: n=80, DE: n=150, IT: n=54, SP: n=43, UK: n=34, US: n=80) who completed a PSC (Table 1). At data collection, mean (standard deviation) age was 64.6 (12.4) years, 36% of patients were female, 19% working full- or part-time and 81% were on second line or later. 8%, 24%, 28% and 40% were at Ann Arbor disease stage I, II, III and IV respectively at the time of data collection.
Overall, 43% of patients agreed that they would be willing to wait longer (up to 4 weeks) for a more effective tx (e.g. CAR-T) than a tx with no delays (not have to wait 4 weeks), whilst 41% of patients agreed that they would be willing to wait longer (up to 4 weeks) for a tx (e.g. CAR-T) if they were less likely to suffer side effects (SEs) than a tx with no delays. 32% and 31% of patients agreed that they would find being hospitalized (for a period of 1-2 weeks) for safety monitoring at the start (first 1-2 months) of their tx acceptable if they did not have to wait longer to start tx (up to 4 weeks) or if they were less likely to suffer SEs respectively. Finally, 37% of patients agreed that they would find being hospitalized (for a period of 1-2 weeks) for safety monitoring acceptable provided the tx was more effective (Table 1).
Conversely, 9% of patients disagreed with the statement "I would find taking a continuous tx as an outpatient for my lymphoma acceptable". 20% of patients disagreed with the statement "I would find being hospitalized for monitoring (for a period of 1-2 weeks) at the start (first 1-2 months) of my tx acceptable if it were more effective". 17% of patients disagreed with the statement "I would find being hospitalized for monitoring (for a period of 1-2 weeks) at the start of my tx acceptable if it had less SEs". 9% of patients disagreed with the statement "I would be willing to wait longer (up to 4 weeks) for a tx (e.g. CAR-T) that was more effective than a tx with no delays" and 11% of patients disagreed with the statement "I would be willing to wait longer (up to 4 weeks) for a tx (e.g. CAR-T) if I were less likely to suffer SEs than a tx with no delays". Finally, 13% of patients disagreed with the statement "I would find being hospitalized (for a period of 1-2 weeks) for monitoring at the start (first 1-2 months) of my tx acceptable if I did not have to wait longer (up to 4 weeks) to start my tx" (Table 1).
Conclusion: A substantial proportion of patients reported that the requirement to wait longer and need for hospitalization were of low concern for their tx selection, often reporting in favor of extended hospitalization if better tx efficacy and SE profile could be achieved.
Quek: Regeneron Pharmaceuticals Inc.: Current Employment, Current holder of individual stocks in a privately-held company. Ma: Regeneron Pharmaceuticals Inc.: Current Employment, Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal